FDA Approves First-Ever Treatment for Children with Menkes Disease
Posted 12 hours ago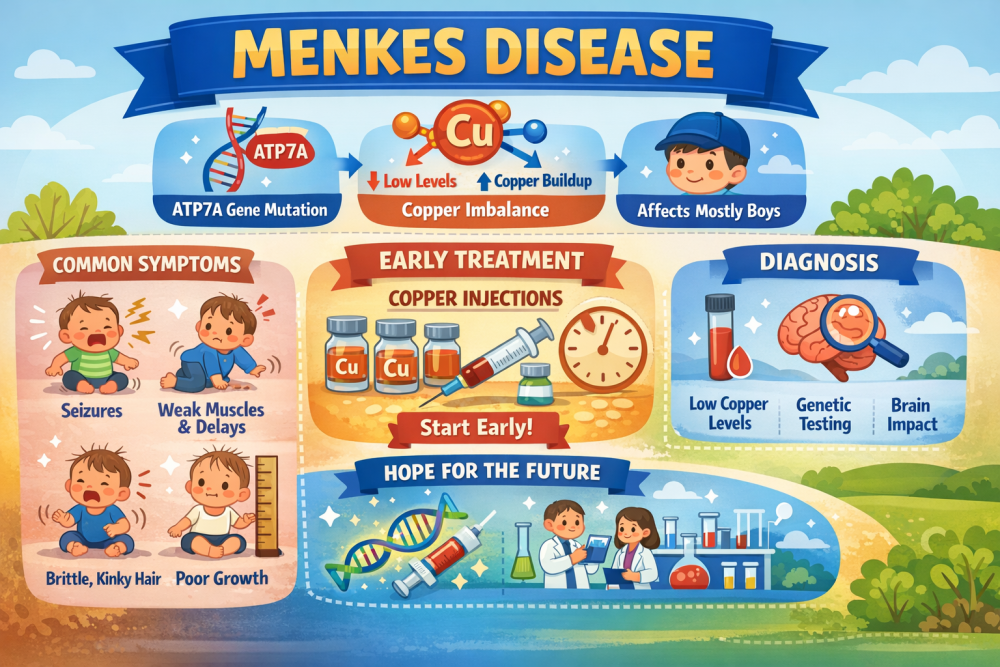
7/2026
The above image has been generated by Scholar GPT 5.2
In January 2026, the U.S. Food and Drug Administration (FDA) approved Zycubo (copper histidinate) injection, making it the first treatment authorized for children with Menkes disease, a rare and often fatal neurodegenerative disorder of infancy.
What is Menkes disease?
Menkes disease is a rare genetic disorder that hinders the body's ability to absorb and distribute copper, a mineral vital for brain development, nerve function, strong bones, and healthy blood vessels. It is caused by mutations in the ATP7A gene, which interfere with copper transport in the body. The condition is estimated to occur in approximately 1 in 100,000 to 250,000 newborns globally and predominantly affects males.
The disease symptoms usually appear in infancy and include:
- Seizures
- Poor growth and weight gain
- Developmental delays and intellectual disability
- Weak muscles and fragile bones
- Abnormal hair that is sparse, brittle, or kinky
Without treatment, children with the most common type classical Menkes disease often do not survive past early childhood.
Why the FDA approval matters
The FDA’s approval of Zycubo is the first FDA-approved therapy for Menkes disease. According to this regulatory body, the decision provides children “the potential to live longer” and marks an unprecedented breakthrough for an ultra-rare disease.
Zycubo is a copper replacement therapy administered by subcutaneous injections. Significantly, it provides copper in a form that bypasses the child’s genetic defect in intestinal copper absorption, enabling the body to utilize the mineral more efficiently.
Evidence behind the decision to Approve Zycubo
The FDA approved it based on data from two open-label clinical trials involving 66 children treated, compared with 17 untreated patients from control groups. The results showed a significant survival benefit.
- Children who began treatment within four weeks of birth had a 78% reduced risk of death compared to untreated children.
- Nearly half of the patients treated early lived longer than six years, and some survived over 12 years.
- None of the untreated patients survived past six years.
Even children who began treatment after 4 weeks of age experienced meaningful improvements in survival.
Safety and monitoring
Like any medication, Zycubo may cause side effects. The most common include infections, respiratory problems, seizures, vomiting, fever, anemia, and injection-site reactions. Because excess copper can build up in the body, the FDA emphasizes the importance of close monitoring during treatment.
A broader impact on rare diseases
The FDA highlighted that Zycubo received several special designations, including Priority Review, Fast Track, Breakthrough Therapy, and Orphan Drug status, reflecting the seriousness of Menkes disease and the urgent unmet medical need.
This approval demonstrates how innovative trial designs and close collaboration with the rare disease community can lead to meaningful treatments, even for conditions affecting only a small number of patients worldwide.
Additional Readings
References
Disclaimer:
The contents provided on www.biomedglobal.org are intended for general informational and educational purposes only. This website's information, articles, and resources are based on data and findings from scientific publications, publicly available research, and reputable sources.
While every effort is made to ensure accuracy and reliability, Biomed Global does not guarantee, endorse, or assume responsibility for the information's completeness, timeliness, or validity.
The material on this website should not be considered a substitute for professional advice, diagnosis, or treatment. Users are strongly encouraged to consult qualified healthcare professionals, researchers, or subject-matter experts before making decisions based on the information presented here.
Under no circumstances shall Biomed Global, its affiliates, contributors, or authors be held liable for any direct, indirect, incidental, or consequential damages arising from the use of, or reliance on, the content of this website.
By accessing and using www.biomedglobal.org you fully acknowledge and agree to this disclaimer.





